Unlike a Mixture a Pure Substance Has
Carbon dioxide CO 2. A pure substance is made up of same kinds of molecules elements and compounds are the basic examples of such matter whereas mixture is made up of two different kinds of molecules homogeneous mixtures and heterogeneous mixtures are the major types of.

Elements Compounds And Mixtures Flashcards Quizlet
Matter made up of two or more different types of particles each with their own.
. Pure Substance and Mixture. Matter can be classified into two broad categories. Molecules can be regarded as the combination of one or more atoms.
Which of the following is an example of a chemical change. Difference Between Pure Substance and Mixture Composition. The matter can be further split into two main categories pure substance or mixture.
Is flour a pure substance or mixture. There is a single type of molecule present in a pure substance unlike mixture. The different substances in a mixture.
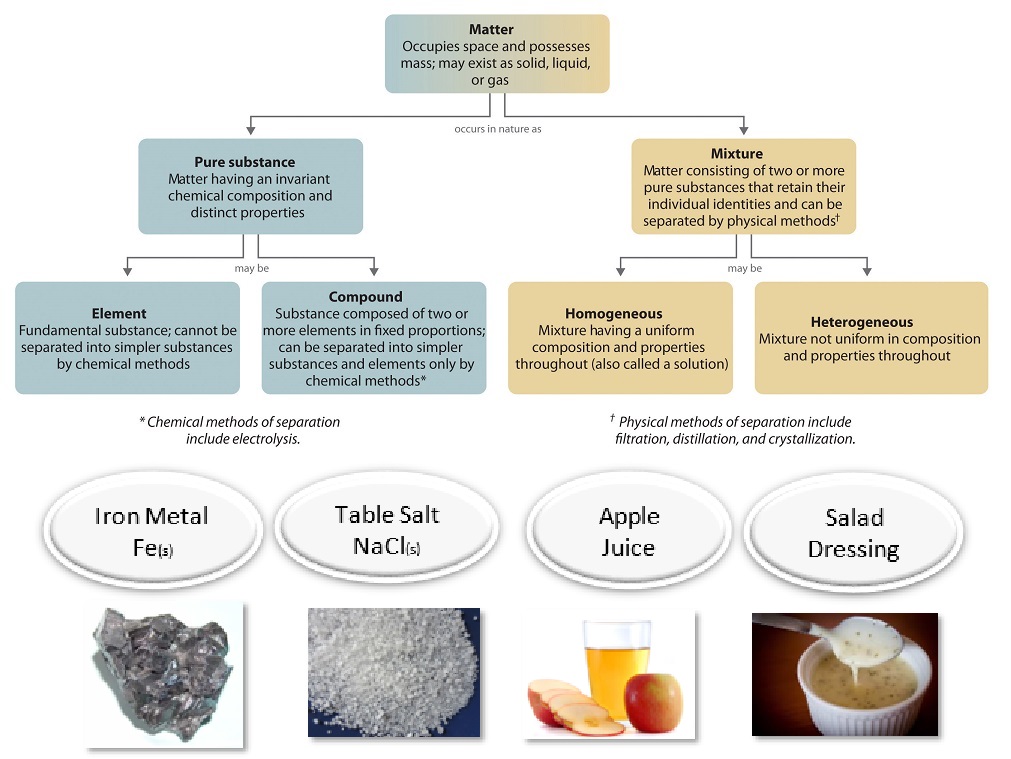
Pure substances A pure substance has a definite and constant composition like salt or sugar. It cannot be broken down or separated into new products. Substances which have a specific composition and cannot be separated into any constituents are.
You cannot notice that is made up of different substances. Tap again to see term. Molecules can be regarded as the smallest particle of a substance which posses the physical as well as chemical propertie s of that particular substance.
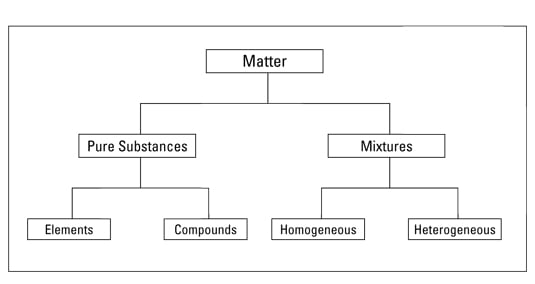
Matter made up of only one type of particle that has the same properties throughout the sample. The matter is divided into two basic categories as pure substance and mixtures. A pure substance contain only a single type of molecule.
The physical and chemical. Unlike a mixture a pure substance has. Whereas a compound may have very different properties from the elements that compose it in mixtures the substances keep their individual properties.
A pure substance is a form of matter that has a definite constant composition and distinctive properties. Unlike a mixture a pure substance has what. Tap card to see definition.
Unlike a mixture a pure substance has. In a heterogenous mixture it is possible to see the various components. A homogeneous mixture looks like it is just one substance.
Unlike a mixture a pure substance has. Mixtures are made up of several substances that are not chemically bonded. A Mixture is made up of a combination of two or more substances that are not united using a chemical reaction.
Which property of a substance is not affected by physical changes. The common salt that we have at home is a compound substance that can be obtained quite pure. Pure substances and mixtures.
Mixtures are physical combinations of two or more elements andor compounds. The physical and chemical properties of pure substances are non-changing if it is on its own without disturbing. Mixtures can always be separated again into component pure substancesbecause bonding among the atoms of the constituent substances does not occur in a mixture.
A pure substance is heterogeneous. The pure substances are further subdivided into elements and compounds. It is made up of two elements.
Mixtures can be seperated by physical means. You can tell it is made up of more than one substance. Material Material Pure Substance or Mixture Element Compound Homogeneous Heterogeneous concrete Mixture Heterogeneous sugar pure water C 12 H 22 O 11 H 2 O Mixture Compound iron filings Fe Pure Substance Element limestone CaCO 3 Pure Substance Element.
It can be. An example is milk or Gatorade. Click again to see term.
There can be one or more types of atoms or molecules in the composition of the chemical compound. Pure substances can be elements made up exclusively of one kind of atom or they can be compounds made up of molecules that. It is the gas that we expel after respiration and that plants require for the photosynthesis process.
A Pure Substance is matter which cannot be separated into its basic components by using a physical or a chemical process. The major difference between pure substances and mixture is that pure substances have a specific composition of constituent while a mixture is the combination of two or more pure substances. Mixtures are physically combined substances that can easily be separated into their primary substances.
PINEAPP PINEAPP 10232017 Chemistry Middle School answered How is a pure substance different from a mixture. The answer is A. The science of what matter is made of and how it changes is called.
An example is sand at the bottom of a beaker of water. A pure substance contain only a single type of molecule. Elements An element is composed of a single kind of atom.
Mixtures can be separated by physical meansA pure substance cannot be separated. Pure substances are made up of mixtures. A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample.
Mixtures can be classified as homogeneous or heterogeneous. On the other hand a mixture is a combination of two or more substances in which they retain their properties. Thus the composition is the same throughout.
On the other hand when we add it to the soup it will be part of a rather complex mixture. A pure sub that cant be broken down into simpler substances is a. A pure substance can be either an element or a compound but the composition of a pure substance doesnt vary.
Pure substances are made of only one matter. It is a mixture It is a pure substance It is a pure substance. Pure substances can be regarded as substances which.
Tap card to see definition. Chemical and physical properties are constant. Click card to see definition.
Any mixture can be formed and then separated by physical means into its pure components without changing the identity of those components. An atom is the smallest particle of an element that still has all the properties of the element.
What Is The Difference Between A Compound And A Mixture Quora
3 5 Pure Substances And Mixtures Chemistry Libretexts
Difference Between Pure Substance And Mixture Definition Composition Properties Examples
0 Response to "Unlike a Mixture a Pure Substance Has"
Post a Comment